Salt Crystals
Prices subject to change without prior notice.
Click on an image to enlarge.
Click on a part number to view instructions
| Kit Name | Part # INSTRUCTIONS/LAB ACTIVITES | Description | Price US $ |
CALCIUM FLUORIDE (FLUORITE)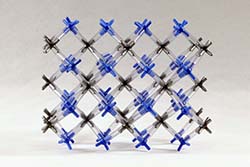 | CAF-1 | Kit Contents: 24 silver 8-peg calcium ion centers 49 blue 4-peg fluoride ion centers 130-1.25" PVC, clear bonds Assembly instruction booklet In the fluorite structure, calcium ions lie in an expanded face centered cube, with fluoride ions filling tetrahedral holes between the cation layers. The coordination number is 4 for the anion and eight for the cation. The model can be used to show these structural arrangements. | $36.40 |
CESIUM CHLORIDE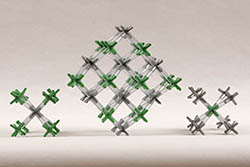 | CsCl-1 | Kit Contents: 26 silver 8-peg cesium ion centers 28 green 8-peg chloride ion centers 97-1.25" PVC, clear bonds Assembly instructions PDF on this website. The model exemplifies the cesium chloride type of ionic structure. Cesium and chloride ions both have a coordination number of eight in this crystal, and its unit cell is simple cubic. | $32.75 |
SODIUM CHLORIDE | SOD-1 | Kit Contents: 40 silver 6-peg sodium ion centers 40 green 6-peg chloride ion centers 158-1.25" PVC, clear bonds Assembly instruction PDF on this website. The kit demonstrates the halite, or rock salt ionic structure. The unit cell (cubic face centered for both the sodium and chloride ions) is easily observed using this model. The coordination number of 6, for cation and for anion, is also easy to demonstrate. | $48.10 |
ZINC SULFIDE (SPHALERITE, OR ZINC BLENDE) | ZNS-1 | Kit Contents: 50 silver 4-peg zinc ion centers 50 yellow 4-peg sulfide ion centers 140-1.25" PVC, clear bonds Assembly instruction booklet Zinc sulfide forms two different mineral forms, sphalerite and wurtzite. With a coordination numbers of 4, 4, the model is also illustrative of an anionic face centered cube with the cations in tetrahedral holes. | $41.85 |
ZINC SULFIDE (WURTZITE)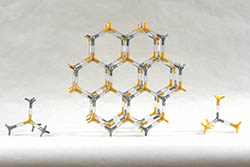 | ZNS-2 | Kit Contents: 43 silver 4-peg zinc ion centers 43 yellow 4-peg sulfide ion centers 122-1.25" PVC, clear bonds Assembly instructions PDF on website Zinc sulfide forms two different mineral forms, sphalerite and wurtzite. Like sphalerite, wurtzite has 4,4-coordination. Our wurtzite model makes the hexagonal, close packing arrangement easy to observe. | $36.45 |
